
Which proteins contain extra amino acids that their genes don’t code for?
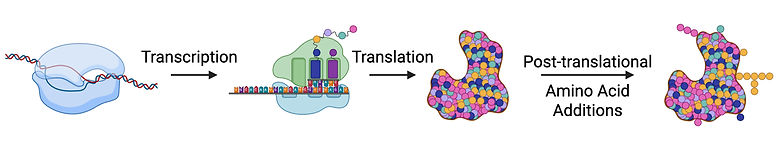
The molecular script of life as we know it lies in DNA, yet my doctoral work shows that not all proteins strictly follow their nucleotide-encoded instructions. While the Central Dogma of Molecular Biology states that a protein’s amino acid sequence is dictated by its gene, post-translational modifications can add amino acids to proteins beyond those encoded. To what extent, and why?
I study proteins that challenge the Central Dogma, designing and applying assays to detect amino acids absent from the DNA sequence in proteins. My goal is to lead a tool-driven research laboratory focused on post-translational amino acid additions, built on two pillars—technological expansion and biological advances.
On this page, you'll find some of my early discoveries which tuned my scientific mind to the nanoscale and fueled my drive to challenge dogmatic claims, and the upcoming work on post-translational amino acid addition that I've been developing during my doctoral training.
The image above this text was generated with Biorender.

Development of a proteomic platform for site detection of post-translational amino acid additions (manuscript in preparation)
Identifying proteins with extra amino acids is hard because these modifications are rare, chemically diverse, sometimes labile, and easily mistaken for isobaric PTMs or sequencing artifacts—plus selective enrichment reagents and tailored MS search strategies have been limited. To address this, I developed a proteomics platform that selectively enriches peptides that are post-translationally modified with amino acids and enables confident, site-level detection across the proteome (manuscript in preparation). The image on the left was generated using Biorender
What's known about post-translational amino acid addition to date? (invited Review - in preparation)
Proteins don’t always follow the DNA script: cells can add extra amino acids to finished proteins, a ribosome-independent process first seen in the 1960s by Hideko and Akira Kaji and now reported for all 20 standard amino acids across bacteria and humans. This review distills 60+ years of work on the chemistries and enzymes behind this unique post-translational modification, what it means for health and disease, explores the question of why evolution kept this second route, and how targeting its “writers” and “erasers” could open new therapies. The image on the right was generated using Biorender

ZNF280A links DNA double-strand break repair to human 22q11.2 distal deletion syndrome
Here we found that a little-known protein called ZNF280A is crucial for fixing dangerous DNA breaks because it helps bring the right repair tools to the damage. People who are missing one copy of this gene (as in 22q11.2 distal deletion syndrome) have weaker DNA repair and more genetic error, but putting ZNF280A back restores proper repair.
Hydrogen Donation but not Abstraction by a Tyrosine (Y68) during Endoperoxide Installation by Verruculogen Synthase (FtmOx1)
In this manuscript, we rechecked how the fungal enzyme FtmOx1 builds an oxygen bridge in a toxin and found it doesn’t use the previously proposed tyrosine “relay” (Y224). Instead, the enzyme pulls the hydrogen directly, sometimes “rebounding” to a side-product when oxygen is scarce, and a different tyrosine (Y68) supplies the final hydrogen—supporting a new, better-fitting model of how the molecule sits in the enzyme.
α-Amine Desaturation of d-Arginine by the Iron(II)- and 2-(Oxo)glutarate-Dependent l-Arginine 3-Hydroxylase, VioC
Here we found that VioC—an oxygen-using enzyme that normally adds an –OH to L-arginine—does something different with the mirror-image D-arginine: it strips off an amino group and turns it into a ketone. The oxygen in that ketone comes from water, and the reaction goes through a short-lived “iminium” step, pointing to a pathway that creates a carbon–nitrogen double bond before breaking it—supporting similar ideas for related enzymes.


